Distal Symmetric Diabetic Polyneuropathy (DSPN)
Abbreviations
- ADA – American Diabetes Association
- ALA – Alpha Lipoic Acid
- CCM – Corneal Confocal Microscopy
- DM – Diabetes Mellitus
- DN – Diabetic Neuropathy
- DPN – Diabetic Peripheral Neuropathy
- DSPN – Distal Symmetric Diabetic Polyneuropathy
- EMG – Electromyography
- FBC – Full Blood Count
- IENFD – Intraepidermal Nerve Fiber Density
- LFTs – Liver Function Tests
- MNSI – Michigan Neuropathy Screening Instrument
- NCS – Nerve Conduction Study
- NC – Nerve Conduction
- PENS – Percutaneous Electrical Nerve Stimulation
- QDIRT – Quantitative Direct and Indirect Test of Sudomotor Function
- QST – Quantitative Sensory Testing
- QSART – Quantitative Sudomotor Axon Reflex Test
- SFN – Small Fiber Neuropathy
- SNRI – Serotonin-Norepinephrine Reuptake Inhibitor
- SSRI – Selective Serotonin Reuptake Inhibitor
- TCA – Tricyclic Antidepressant
- TENS – Transcutaneous Electrical Nerve Stimulation
- UENS – Utah Early Neuropathy Scale
- UKST – United Kingdom Screening Test .
INTRODUCTION
Diabetic neuropathy is the most common complication of diabetes mellitus (DM), affecting as many as 50% of patients with type 1 and type 2 DM.
A nerve fiber, also called an axon, is a long and slender projection of nerve cells (or neurons) that carry electrical impulses.
- Primarily small-fiber neuropathy
- Primarily large-fiber neuropathy
- Mixed small- and large-fiber neuropathy (most common).
Classification of Nerve Fibers
- Group A Nerve Fibers: Group A nerve fibers are heavily myelinated nerve fibers that are further subdivided into four types: alpha Aα; beta Aβ; gamma Aγ; and delta Aδ. The fibers with larger diameters and more myelination tend to transmit the impulses at a faster rate.
- Group B Nerve Fibers: Group B nerve fibers are less myelinated than group A, but more myelinated than group C nerve fibers. They include visceral nerves such as the vagus nerve.
- Group C Nerve Fibers: Group C nerve fibers are unmyelinated fibers that usually have a smaller diameter and low conduction velocity.
Nerve fibers are classified based on their thickness into small fibers and large fibers depending on how thick they are.
Large fiber nerve cells have diameters more than about 5 micrometers, and small fibers are thinner.
Large myelinated nerve fibers
| Type | Size μm | Function | Myelinated |
| A-alpha | 13-20 | Limb proprioception | Yes |
| A-beta | 6-12 | Fine touch discrimination, vibration, pressure | Yes |
| A-gamma | 2 – 8 | Motor fibers for the muscle spindle, which helps maintain muscle tone and adjust the sensitivity of muscle spindles. | Yes |
Small myelinated nerve fibers
| Type | Size μm | Function | Myelinated |
| C | 0.2-1.5 | Fast pain (sharp, acute pain) and temperature. | No |
RISK FACTORS
| Major Risk Factors | Other Risk Factors |
|
|
CLINICAL MANIFESTATIONS
- Up to one-half of patients with diabetic polyneuropathy may be asymptomatic.
- Long axons are more sensitive to nerve damage and complaints usually begin distally to the lower limbs and more rarely from the distal upper limbs.
- Involvement of myelin free C, thin myelin A delta, thick myelin Aα and Aᵦ type neurons are typical.
- The symptoms are usually exacerbates at night.
- The symptoms and signs are usually symmetric in both sides.
- The symptoms are usually localized distally, starting at big toe and ascend proximally.
- With disease progress slowly, sensory loss ascends and, when reaching approximately mid-calf, appears in the hands.
- This gradual evolution causes the typical “stocking-glove” sensory loss.
- Motor involvement with frank weakness occurs in the same pattern, but only much later and in more severe cases.
Small myelinated nerve fibers
| Symptoms | Examination |
|
1. Neuropathic Pain: Patients may experience sharp, burning, or shooting pain or sharp electric shock-like pains shooting through their limbs. 2. Temperature Sensitivity: intolerance to heat or cold or may feel constantly too hot or too cold. may have difficulty discerning changes in temperature, making them susceptible to burns or frostbite due to an inability to sense heat or cold accurately. 3. Allodynia : There might be a painful response to stimuli that do not normally provoke pain. 3. Hyperalgesia: an increased sensitivity to painful stimuli. 4. Numbness: As the disease progresses, numbness can occur, which may lead to loss of protective sensation, increasing the risk of skin ulcers and infections. 5. Tingling and Prickling or or Crawling Sensations: often described as “pins and needles”. |
Thermal (cold/hot) discrimination: reduced/absent Pinprick sensation: reduced/absent light touch |
Large myelinated nerve fibers
| Symptoms | Examination |
|
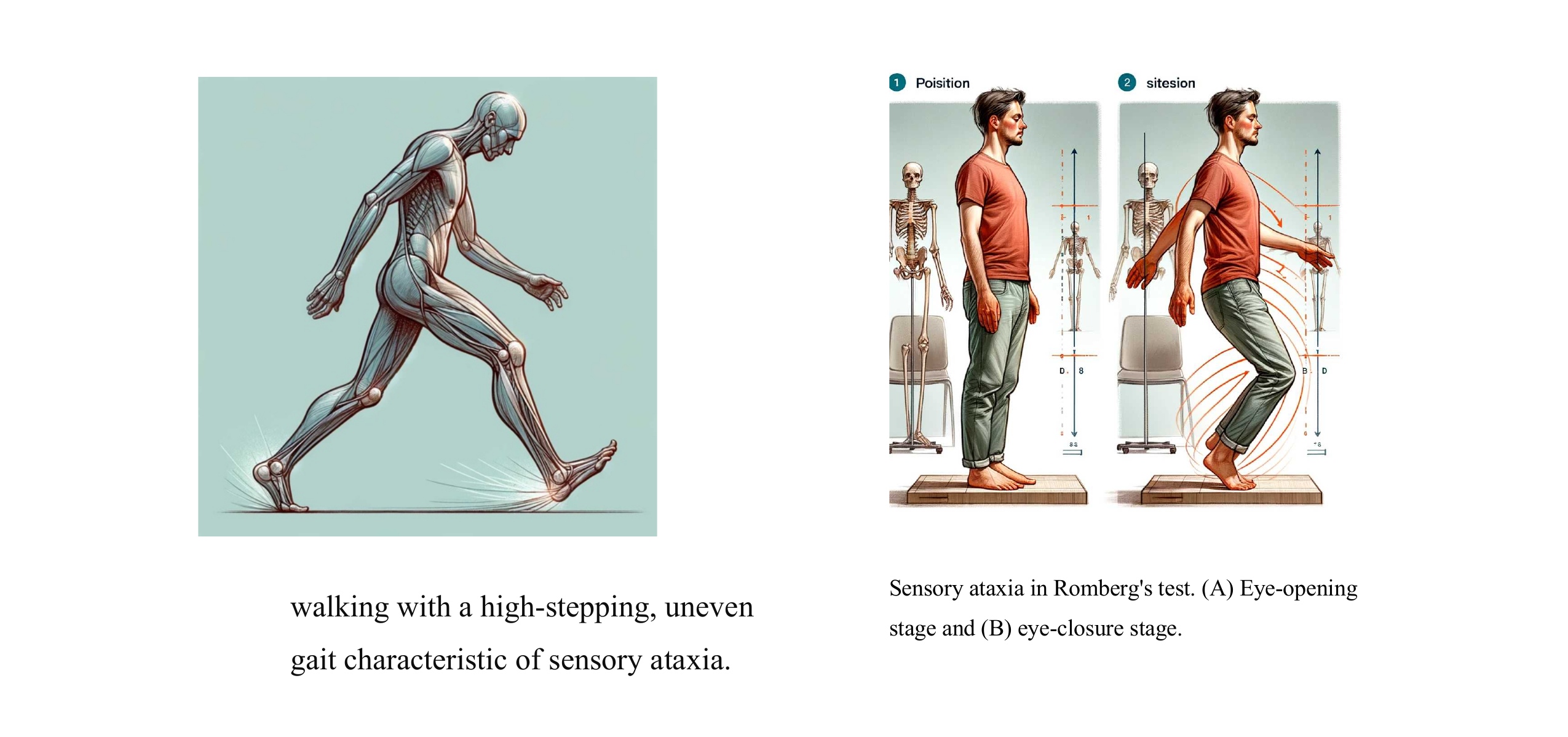
. Muscle Weakness: Damage to large myelinated nerve fibers can result in muscle weakness, particularly in the lower extremities. Patients may experience difficulty with activities that require strength and coordination, such as climbing stairs, standing from a seated position, or lifting objects. Impaired Gait: Weakness and loss of coordination due to motor nerve involvement can contribute to changes in gait patterns. Patients may exhibit an unsteady or shuffling gait, with reduced arm swing and difficulty maintaining balance. Foot Drop: Dysfunction of the peroneal nerve, which innervates the muscles responsible for dorsiflexion of the foot, can lead to foot drop. This condition causes the toes to drag while walking and increases the risk of tripping and falling. Sensory Ataxia: Impaired proprioception and coordination can result in ataxic gait, characterized by unsteadiness, irregular step length, and difficulty maintaining balance. Patients may sway from side to side while walking and exhibit a wide-based stance to compensate for the lack of stability. Painful Neuropathy : Patients may experience deep, aching pain in the affected limbs, which can be exacerbated by movement or pressure. Reduced Touch Sensitivity. Painless Injuries |
Ankle reflexes: reduced/absent Vibration perception: reduced/absent 10 g monofilament: reduced/absent Proprioception: reduced/absent light touch: reduced/absent muscle atrophy Positive Romberg’s test (while closing eyes) |
| Performance of different groups in Romberg’s test. | ||
| Groups | Open eyes stage | Close eyes stage |
| Normal | Stable | Stable |
| Sensory ataxia | Stable | Instable |
| Cerebellar ataxia | Instable | Instable |
DIAGNOSTIC TESTS
Indications:
- Atypical clinical features: (such as asymmetrical symptoms and signs or weakness)
Tests for Small Fibers:
- Quantitative Sensory Testing (QST): This can measure response to pain and temperature changes, typically assessing small fiber function.
- Skin biopsy and Intraepidermal Nerve Fiber Density (IENFD): Directly measures small nerve fibers in the skin via biopsy.
- Corneal Confocal Microscopy (CCM): Visualizes small nerves in the cornea, reflecting changes in small fiber nerve health.
- Quantitative Sudomotor Axon Reflex Test (QSART): Assesses the autonomic nerves that control sweating, linked to small fibers.
Tests for Large Fibers:
- Nerve Conduction Studies (NCS): Measures the electrical conduction ability of large myelinated fibers, providing data on their functional status.
- Quantitative Sensory Testing (QST): Can also be used to measure vibration perception thresholds, thus evaluating large fiber function.
Tests for Both:
- DPNCheck: A hand-held device used to perform nerve conduction studies that can assess both small and large fibers based on the parameters and nerves tested.
DIAGNOSTIC CRITERIA
1. 2017 American Diabetes Association (ADA) position statement:
The diagnosis of diabetic polyneuropathy is based primarily on clinical findings in a patient with diabetes, and one of the following:
- A combination of typical symptoms with typical signs, particularly symmetric distal sensory loss.
- Typical signs on examination in the absence of symptoms or with only the presence of a painless foot ulcer
These ADA criteria can be applied in routine clinical practice.
Note that the diagnosis of diabetic polyneuropathy is one of exclusion.
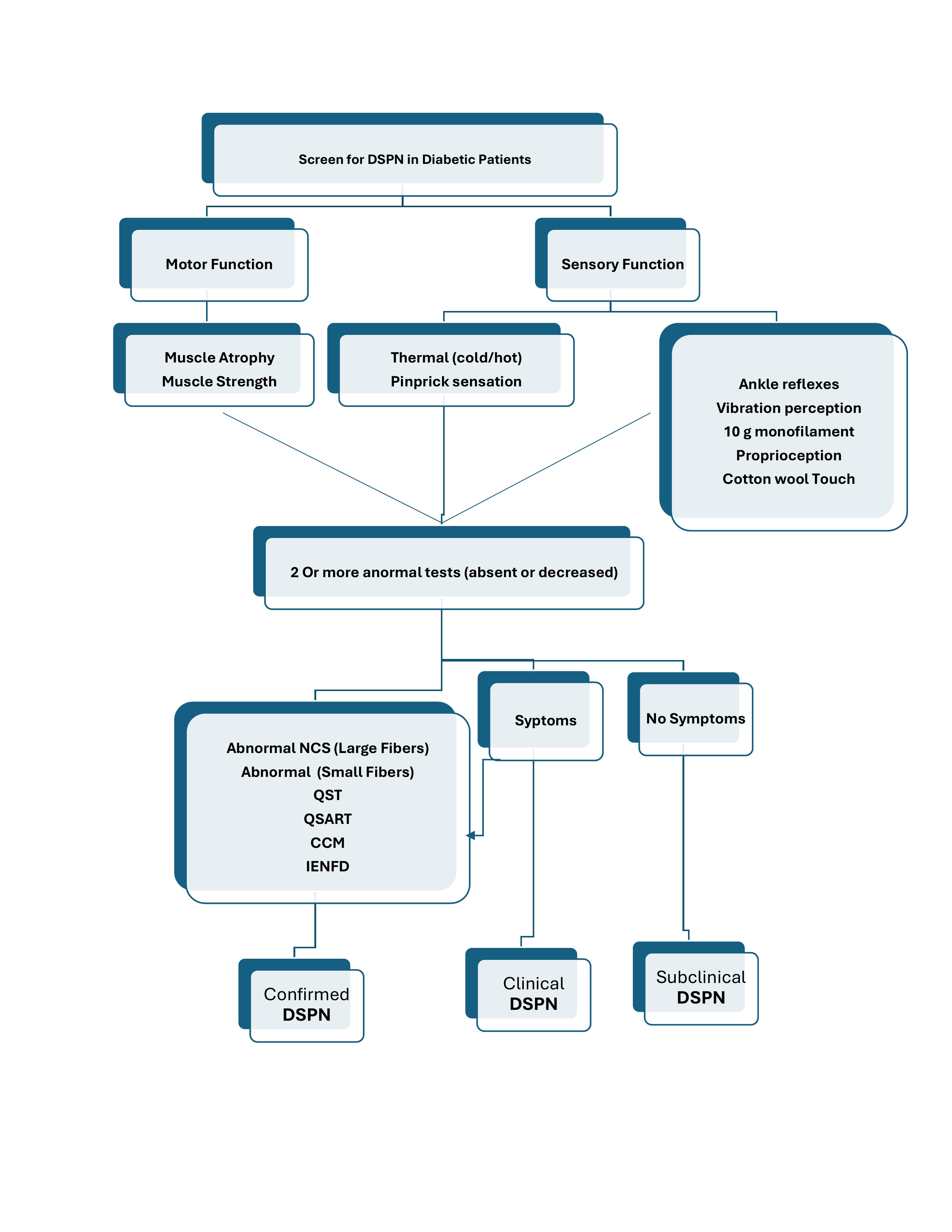
2. Toronto Expert Panel on Diabetic Neuropathy diagnostic criteria for distal symmetrical polyneuropathy (DSPN)
· Possible clinical DSPN
- Presence of symptoms or signs of DSPN..
· Probable clinical DSPN
A combination of symptoms and signs of distal sensorimotor polyneuropathy include any 2 or more of the following:
- Neuropathic symptoms
- Decreased distal sensation
- Unequivocally decreased or absent ankle reflexes.
· Confirmed clinical DSPN
An abnormal nerve conduction study (NCS), and a one or more of symptoms or one or more of signs of sensorimotor polyneuropathy.
If If NC is normal, a validated measure of small fiber neuropathy (SFN) may be used.
· Subclinical DSPN (stage 1a)
- No signs or symptoms of polyneuropathy
- Abnormal nerve conduction.
- Or a validated measure of SFN.
Toronto Expert Panel on Diabetic Neuropathy definition of small fiber neuropathy
o Possible small-fiber neuropathy
Presence of distal symmetrical symptoms, and/or clinical signs of small-fiber damage.
o Probable small-fiber neuropathy
Presence of distal symmetrical symptoms, clinical signs of small-fiber damage, and normal or abnormal sural nerve conduction (NC) study.
o Definite small-fiber neuropathy
Presence of length-dependent symptoms, clinical signs of small-fiber damage,
And one or more abnormal test:
- NC study
- QST
- IENFD
- QSART
- CCM
WORKUP
All patients should be assessed for diabetic peripheral neuropathy (DPN) starting at diagnosis of type 2 diabetes and 5 years after the diagnosis of type 1 diabetes and at least annually thereafter.
STAGING
A common staging scale of diabetic polyneuropathy is as follows:
- NO – No neuropathy
- N1a – Signs but no symptoms of neuropathy
- N2a – Symptomatic mild diabetic polyneuropathy; sensory, motor, or autonomic symptoms; patient able to heel walk
- N2b – Severe symptomatic diabetic polyneuropathy (as in N2a, but patient unable to heel walk)
- N3 – Disabling diabetic polyneuropathy
Staging of Diabetic Peripheral Neuropathy
The staging is often based on clinical findings and symptoms:
-
Stage 0: No Neuropathy
- Clinical Features: No signs or symptoms of neuropathy are present.
- Diagnostic Tests: Normal results in clinical and nerve function tests.
-
Stage 1: Asymptomatic Neuropathy
- Clinical Features: No symptoms, but signs of neuropathy are present, such as diminished reflexes or loss of vibration sensation.
- Diagnostic Tests: Abnormal nerve conduction studies or other diagnostic tests like Quantitative Sensory Testing (QST).
-
Stage 2: Symptomatic Neuropathy
- Clinical Features: Symptoms such as pain, burning, tingling, or numbness are present along with clinical signs of neuropathy.
- Diagnostic Tests: Tests continue to show abnormalities, confirming nerve dysfunction.
-
Stage 3: Advanced Symptomatic Neuropathy
- Clinical Features: Severe symptoms, including constant pain, major sensory loss, or weakness. Patients may also experience complications like foot ulcers due to diminished sensation.
- Diagnostic Tests: Marked abnormalities in nerve function tests, significant loss in nerve fiber density may be noted.
-
Stage 4: Disabling Neuropathy
- Clinical Features: Severe and disabling symptoms with potential for significant motor involvement and major autonomic dysfunction. This stage includes major limb-threatening complications.
- Diagnostic Tests: Profound abnormalities on all neurologic testing, with extensive loss of nerve fibers.
INVESTIGATIONS
1st test to order
| Test | Comment |
| clinical diagnosis | |
| fasting blood glucose | If DM not already known to be present |
| HbA1c | Degree of DM control |
| TSH | |
| serum vitamin B12 | |
| electrolytes, urea, creatinine | |
| serum lipid profile | |
| LFTs | |
| FBC and erythrocyte sedimentation rate | To exclude anaemia and inflammatory disorders. |
| serum/urine immunoelectrophoresis | To exclude multiple myeloma. |
| corneal confocal microscopy | corneal nerve fiberdamage correlates with intra-epidermal nerve fiberloss and severity of neuropathy |
Other tests to consider
| Test | Comment |
| nerve conduction studies | |
| electromyography (EMG) | |
| quantitative sensory testing (QST) | |
| skin biopsy |
COMPLICATIONS
- foot deformities with pes cavus and clawing of the toes:
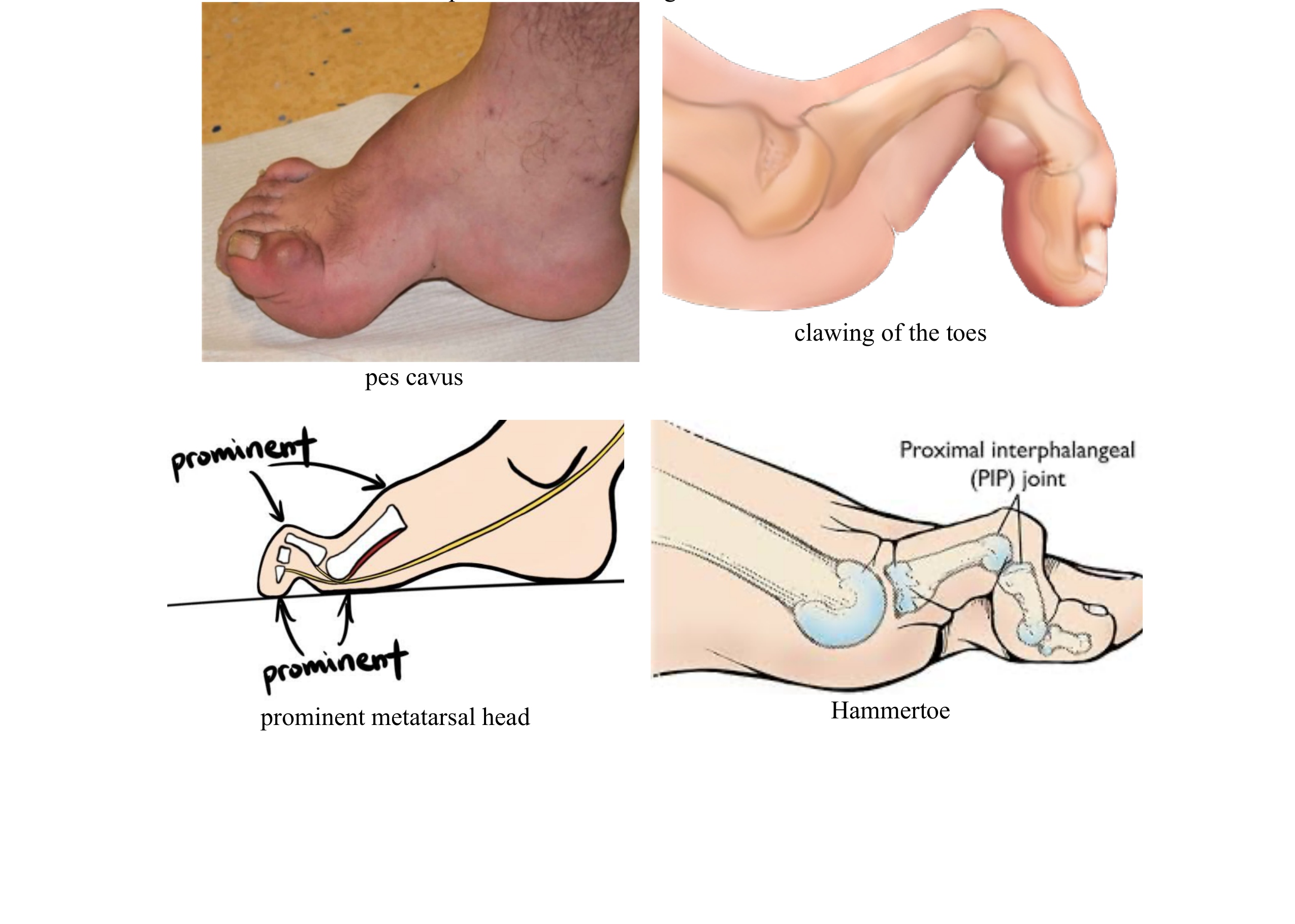
- atrophy and reflex losses may occur in the foot muscles; due to loss of pain sensation and deformities in the feet, prominent metatarsal head, claw-foot, hammertoe deformities are frequently observed.
- These deformations also increase the risk of callus
- chronic neuropathic foot ulcers and amputation.
- Neuropathic pain .
- Charcot neuroarthropathy
APPROACH TO SCREENING
applies primarily to patients with an established diagnosis of diabetes mellitus.
Who should be screened? — Our recommendations agree with those of the 2017 ADA position statement:
- All patients with diabetes should be screened for polyneuropathy at the time of diagnosis of type 2 diabetes and five years after the diagnosis of type 1 diabetes.
- Patients with prediabetes (ie, impaired fasting glucose and/or impaired glucose tolerance) who have symptoms of polyneuropathy should also be screened.
- After initial screening, all patients with type 2 or type 1 diabetes who do not have polyneuropathy should be screened at least annually for the development of neuropathy.
Screening assessment includes the following essential elements:
- A careful history
- Assessment of small nerve fiber function by testing thermal or pin prick sensation and light touch perception with a 10 g monofilament on the dorsal aspect of the distal great toe
- Assessment of large nerve fiber function by testing vibration sensation with a 128 Hz tuning fork, proprioception (joint position sensation), and deep tendon reflexes at the ankle as compared with more proximal locations.
The recommended ADA assessments can be met by using one or more simple screening tests (see ‘Simple screening tests’ below).
The examiner should ask about the following:
| History: | |
| Start of Symptoms | Typically start in the toes |
| Type of Symptoms | Numbness, tingling, and pain are typical early symptoms |
| symptoms worse at night | |
| painful when bedsheets touch the feet | |
| pain stabbing, burning, or lightening-like | |
| Burning pain, allodynia, and nocturnal worsening | are common in diabetic polyneuropathy. |
| any weakness present | Weakness typically develops late and usually first affects toe extension and ankle dorsiflexion. |
| symptom progression | Slow progression is typical |
| symptoms changed over time | Proximal spread is typical. |
| any autonomic symptoms present | Prominent autonomic involvement is atypical, especially early in the disease course. |
| history of alcohol use | Prolonged excessive alcohol use is a common cause of symmetric polyneuropathy. |
| family history of similar symptoms, or a family history of high arches or hammer toes | A family history suggests the possibility of a hereditary neuropathy; high arches or hammer toes suggest Charcot-Marie-Tooth disease. |
| Symmetry of symptoms | Symmetry of symptoms is typical |
| symptoms changed over time | Proximal spread is typical. |
| Present of other medical problems | |
| clinical examination of the feet and legs should be include: | |
| Pinprick sensation at the toes compared with the knees | assess small nerve fiber function |
| temperature sensation at the toes compared with the knees | |
| Proprioception at the toes | assess large nerve fiber function |
| Vibration perception | |
| 10 g monofilament | |
| Ankle and patellar deep tendon reflexes | |
| Cotton wool Touch | |
| Romberg test, normal gait, and tandem gait | assess balance and fall risk |
Simple screening tests:
1. Michigan Neuropathy Screening Instrument (MNSI) form
| A. History (to be completed by the person with diabetes) | ||
| Are your legs and/or feet numb? | 1 Yes | 0 No |
| Do you ever have any burning pain in your legs and/or feet? | 1 Yes | 0 No |
| Are your feet too sensitive to touch? | 1 Yes | 0 No |
| Do you get muscle cramps in your legs and/or feet? | 1 Yes | 0 No |
| Do you ever have any prickling feelings in your legs or feet? | 1 Yes | 0 No |
| Does it hurt when the bed covers touch your skin? | 1 Yes | 0 No |
| When you get into the tub or shower, are you able to tell the hot water from the cold water? | 0 Yes | 1 No |
| Have you ever had an open sore on your foot? | 0 Yes | 1 No |
| Has your doctor ever told you that you have diabetic neuropathy? | 0 Yes | 1 No |
| Do you feel weak all over most of the time? | 0 Yes | 1 No |
| Are your symptoms worse at night? | 0 Yes | 1 No |
| Do your legs hurt when you walk? | 0 Yes | 1 No |
| Are you able to sense your feet when you walk? | 0 Yes | 1 No |
| Is the skin on your feet so dry that it cracks open? | 1 Yes | 0 No |
| Have you ever had an amputation? | 1 Yes | 0 No |
| Total score:* __________ | ||
Patients with a total score >4 points are considered to have neuropathy.
| B. Physical assessment (to be completed by health professional) | ||||||||
| Right | Left | |||||||
| Appearance of foot | Appearance of foot | |||||||
| Normal | 0 Yes | 1 No | Normal | 0 Yes | 1 No | |||
| For any of the following: | For any of the following: | |||||||
| Deformities | 0 No 1 Yes | Deformities | 0 No 1 Yes | |||||
| Dry skin, callus | Dry skin, callus | |||||||
| Infection | Infection | |||||||
| Fissure | Fissure | |||||||
| Other | Other | |||||||
| Specify: ____________________ | Specify: ____________________ | |||||||
| Ulceration | Ulceration | |||||||
| Absent 0 |
Present 1 |
Absent 0 |
Present 1 |
|||||
| Ankle reflexes | Ankle reflexes | |||||||
| Present 0 |
Present/reinforcement 0.5 |
Absent 1 |
Present 0 |
Present/reinforcement 0.5 |
Absent 1 |
|||
| Vibration perception at great toe | Vibration perception at great toe | |||||||
| Present 0 |
Decreased 0.5 | Absent 1 |
Present 0 |
Decreased 0.5 | Absent 1 |
|||
| Thermal Or Pin Prick at great toe** | Thermal Or Pin Prick at great toe** | |||||||
| Present 0 |
Absent 1 |
Present 0 |
Absent 1 |
|||||
| Total score: __________ /10 points | ||||||||
| Signature: _____________________________________________ | ||||||||
A physical assessment score ≥2.5 indicates a diagnosis of clinical neuropathy with a sensitivity and specificity of 61 and 95 percent, respectively.
** Added to align with the 2017 ADA guidelines.
- Utah Early Neuropathy Scale (UENS)
The Utah Early Neuropathy Scale (UENS) is a simple screening tool that assesses both large and small fiber nerve function, and aligns with the 2017 ADA recommendations.
A score of ≥4 defines clinical neuropathy.
3. United Kingdom Screening Test (UKST)
| Symptom Score | |||
| Burning, numbness, or tingling in the feet | 2 Yes | 0 No | Max 2 points |
| Fatigue, cramping, or aching | 1 Yes | 0 No | |
| Location of symptoms | 2 Feet | 1 Calves | 0 Elsewhere |
| Awakened you at night? | 1 Yes | 0 No | |
| Timing of symptoms | Worse at night 2 | Day& Night 1 | Day only 0 |
| Symptoms relieved by | 2 Walking | 0 Standing | 0 Sitting or lying or No relief |
|
0 to 2 points: Normal 3 to 4 points: Mild polyneuropathy 5 to 6 points: Moderate polyneuropathy 7 to 9 points: Severe polyneuropathy |
Total score:* __________ | ||
| Physical Findings | |||||||
| Right | Left | ||||||
| Ankle reflexes | Ankle reflexes | ||||||
| Present 0 |
Present/reinforcement 1 |
Absent 2 |
Present 0 |
Present/reinforcement 1 |
Absent 2 |
||
| Vibration Sense | Vibration Sense | ||||||
| Present 0 |
Decreased/Absent 1 |
Present 0 |
Decreased/Absent 1 |
||||
| Pin Prick Sensation | Pin Prick Sensation | ||||||
| Present 0 |
Absent 1 |
Present 0 |
Absent 1 |
||||
| Temperature Sensation | Temperature Sensation | ||||||
| Present 0 |
Decreased 1 |
Present 0 |
Decreased 1 |
||||
|
Results |
|
|
0 to 2 points: Normal 3 to 5 points: Mild polyneuropathy 6 to 8 points: Moderate polyneuropathy 9 to 10 points: Severe polyneuropathy |
Score: |
| Peripheral polyneuropathy is present if | High Risk for Ulceration |
|
Physical Signs Score (≥6 points) Or Physical Signs Score (≥3 points) and Symptoms Score (≥5 points). |
Physical Signs Score (≥8 points) |
COMMON CAUSES OF DSPN
| Diseases | Comment |
| Metabolic | |
| Diabetes | Most common cause, accounting for 32 to 53% of cases* |
| Prediabetes | Glucose tolerance test has highest sensitivity* |
| Chronic kidney disease | Neuropathy particularly severe when chronic kidney disease is caused by diabetes |
| Chronic liver disease | Neuropathy typically mild |
| Idiopathic | 24 to 27% of all cases* |
| Toxin (alcohol) | Second most common cause (requires in-depth questioning)* |
| Inherited | Detailed family history required; ask about hammer toes, high arches* |
| Charcot-Marie-Tooth disease type 1 | Inherited demyelinating sensory motor neuropathy |
| Charcot-Marie-Tooth disease type 2 | Inherited axonal sensory motor neuropathy |
| Familial amyloidosis | Transthyretin mutation most common |
| Nutritional | |
| Vitamin B12 deficiency | Methylmalonic acid level important when vitamin B12 level is 200 to 400 picogram/mL* |
| Vitamin E deficiency | Can cause cerebellar ataxia |
| Vitamin B6 deficiency | Can cause neuropathy when level is too high or too low |
| Thiamine deficiency | Can present with ataxia, ophthalmoparesis, and confusion |
| Copper deficiency | Often presents with a myeloneuropathy |
| Gastric bypass surgery | Often difficult to determine which factor responsible |
| Malabsorption syndromes | Often difficult to determine which factor responsible |
| Medication | |
| Chemotherapy (vincristine, cisplatin, taxol, bortezomib) | Known dose limiting side effect of many agents |
| Amiodarone | Can cause a demyelinating neuropathy |
| Phenytoin | Typically after many years of use |
| Nucleosides | Can be difficult to distinguish cause of neuropathy (HIV versus medication) |
| Nitrofurantoin | Worse in the setting of kidney failure |
| Metronidazole | Usually after high, prolonged intravenous doses |
| Hydralazine | Avoid by concomitant use of vitamin B6 |
| Isoniazid | Avoid by concomitant use of vitamin B6 |
| Colchicine | Can also cause myopathy |
| Autoimmune | |
| Rheumatoid arthritis | Can also cause mononeuritis multiplex |
| Lupus | Can also cause mononeuritis multiplex |
| Sjögren syndrome | Can also cause a sensory neuronopathy or mononeuritis multiplex |
| Sarcoidosis | Can present with several neurologic manifestations |
| Secondary amyloidosis | Diagnosis aided by fat pad biopsy or sural nerve biopsy |
| Infectious | |
| HIV | Medications used to treat can also cause neuropathy |
| Hepatitis B/C | Can also cause mononeuritis multiplex associated with polyarteritis nodosa and cryoglobulinemia |
| Neoplastic | |
| Monoclonal gammopathy of unclear clinical significance | Immunofixation increases sensitivity of paraprotein detection* |
| Multiple myeloma | Associated with IgG or IgA paraproteinemia |
| Primary amyloidosis | Diagnosis aided by fat pad biopsy or sural nerve biopsy |
IgA: immunoglobulin A; IgG: immunoglobulin G.
MANAGEMENT
1. Preventive Care:
Glycemic control
Risk factor modification
Lifestyle interventions are endorsed as an essential practice to prevent onset and progression of neuropathy, particularly in individuals with prediabetes and type 2 diabetes.
Goals include achieving a normal bodyweight and attaining individualized glycemic, blood pressure, and lipid goals along with 150 minutes of moderate-to-vigorous aerobic activity and two to three sessions of resistance training weekly.
Foot care
Peripheral neuropathy is one of the most important risk factors for ulcers and amputations in patients with diabetes. Foot care in patients with neuropathy is essential to help lower risk of complications.
On a daily basis, patients should inspect their feet for the presence of dry or cracking skin, fissures, plantar callus formation, and signs of early infection between the toes and around the toenails. Regular clinical foot examinations to detect peripheral neuropathy are also an essential component of preventive care in all patients with diabetes.
Safety and falls
Patients with diabetic neuropathy are at increased risk for gait instability and falls due to progressive loss of proprioception, foot pain, orthostatic hypotension due to autonomic dysfunction, age-related functional impairments, and medication side effects. Although not well studied in this specific patient population, multifaceted interventions including home-based exercise, physical and occupational therapy, and home safety evaluation have been shown to reduce fall risk in older adults and should be considered for patients with gait and balance abnormalities on neurologic examination.
B12 deficiency
Metformin reduces intestinal absorption of B12, and the prevalence of borderline or low B12 levels in metformin-treated patients approaches 20 percent at five years . Patients on metformin with worsening neuropathy symptoms should be screened for B12 deficiency, particularly if examination findings are atypical or suddenly worsening.
2. Pain Management:
In the UK, the National Institute of Health and Care Excellence (NICE) recommends the
- gabapentinoids (pregabalin and gabapentin).
- SNRI (duloxetine).
- tricyclic antidepressant (amitriptyline).
Neuropathic Pain Special Interest Group resulted in a strong recommendation for first-line treatment with
- SNRIs.
- pregabalin, gabapentin.
- tricyclic antidepressants.
American Academy of Neurology Level of Recommendation
- Pregabalin, gabapentin,
- SNRIs
- TCAs
Neuropathic Pain Management Algorithm
| Neuropathic Pain Management | ||
| Step 1 |
Glycemic control Risk factor modification Foot care Safety and falls |
|
|
TCAs (Tricyclic Antidepressants) Or SNRIs (Serotonin-Norepinephrine Reuptake Inhibitors) Or Gabapentinoids |
||
| Step 2 | Pregabalin and SNRIs (Duloxetine and Pregabalin) Pregabalin and TCAs (Amitriptyline with Pregabalin) or (Imipramine and Pregabalin) | OR add ALA (Alpha Lipoic Acid) |
| Step 3 |
Pregabalin and SNRIs and TCAs (Pregabalin with Amitriptyline, and Duloxetine) Refer to Multidisciplinary Pain ClinicReassess for Mood Disorder |
OR add ALA (Alpha Lipoic Acid) |
| Other Treatments | Topical therapies | topical capsaicin or Lidocaine patches glyceryl trinitrate |
| Sodium-channel blockers | Valproic acid Carbamazepine | |
| Selective serotonin-reuptake inhibitors (SSRIs) | paroxetine Fluoxetine Sertraline | |
| cognitive behavioural therapy (CBT) | ||
| Transcutaneous electrical nerve stimulation (TENS) | ||
| Percutaneous electrical nerve stimulation (PENS) | ||
| Acupuncture in refractory cases | ||
| Spinal cord stimulation | For refractory to all treatments | |
Neuropathic Pain Medications
| Class | Drug Name | Usual Dose |
| Gabapentinoids | Gabapentin | 300 – 1200 mg three times a day |
| Pregabalin | 150-600 mg/d in two to three divided doses | |
| SNRIs | Duloxetine | 30 mg/d to 60 mg two times a day |
| Venlafaxine | 75-225 mg orally (extendedrelease) once daily | |
| Desvenlafaxine | 50-400 mg daily | |
| Levomilnacipran | 40-120 mg daily | |
| Milnacipran | 50 mg twice daily | |
| TCAs | Amitriptyline | Amitriptyline, 25–75 mg orally at bedtime |
| Nortriptyline | 10-25 mg orally once daily at bedtime initially, increase according to response, maximum 150 mg/day | |
| Imipramine | 10-25 mg orally once daily at bedtime initially, increase according to response, maximum 150 mg/day | |
| Desipramine | 25–150 mg/day orally | |
| Others | Alpha-lipoic acid (ALA) | oral ALA (600 mg daily). |
| Topical therapies | capsaicin topical | (0.025% or 0.075%) apply to the affected area(s) up to four times daily when required |
| glyceryl trinitrate | 0.4 mg/dose spray | |
| Lidocaine patches (5%) | Patches may remain in place for no more than 12 hours in any 24-hour period | |
| Sodium-channel blockers | Valproic acid | 500 to 1200 mg daily |
| Carbamazepine | ||
| SSRI | paroxetine | 40 mg orally once daily |
